In the last decade, there has been a marked increase in the number of prostate cancer treatments being developed, as well as the speed in which they are trialed and approved. Although prostate cancer continues to be the second leading cause of cancer death in men in the U.S.1, these treatment advancements are improving the quality of care and long-term survival rates for these men.
While the suppression of testosterone and use of oral oncolytics will be a constant part of treatment for men with castrate resistant prostate cancer, let’s not lose sight of the fact that they are only one piece of the puzzle. The reality is that these methods are only part of the solution for the more than 40,000 men in the U.S. who will see their disease become castrate resistant and metastasize2.
Although metastatic castrate-resistant prostate cancer (mCRPC) is not curable, the number of treatments that have become available in just the last 10 years have given urologists the tools to control the cancer for prolonged periods of time. A paradigm shift in how we treat mCRPC began in 2010 with the introduction of an immunotherapy for mCRPC.
When sipuleucel-T was FDA-approved, it prolonged overall survival (OS) among men with mCRPC by 4.1 months.3 Reported in the pivotal IMPACT trial in the New England Journal of Medicine, this was very significant at a time when patients in the later stages of prostate cancer virtually had no options aside from chemotherapy.
Urology was one of the first specialties to adopt immunotherapy, dating back to the use of Bacillus Calmette-Guérin (BCG) in the late 90s4. While sipuleucel-T has become an increasingly vital cancer treatment option for mCRPC patients, it remains largely underutilized in part because it differs mechanistically from other prevailing forms of cancer treatment that affect familiar prognostic biomarkers, such as PSA.
Instead, sipuleucel-T stimulates the body’s immune system to target and attack prostate cancer cells that express PAP (prostatic acid phosphatase) – a protein present on 95% of prostate cancer cells5. As we come to understand more about how immunotherapy works, research indicates that this type of treatment may have a more profound effect earlier in the disease progression when the tumor volume and associated immune-suppressive mechanisms are lower.
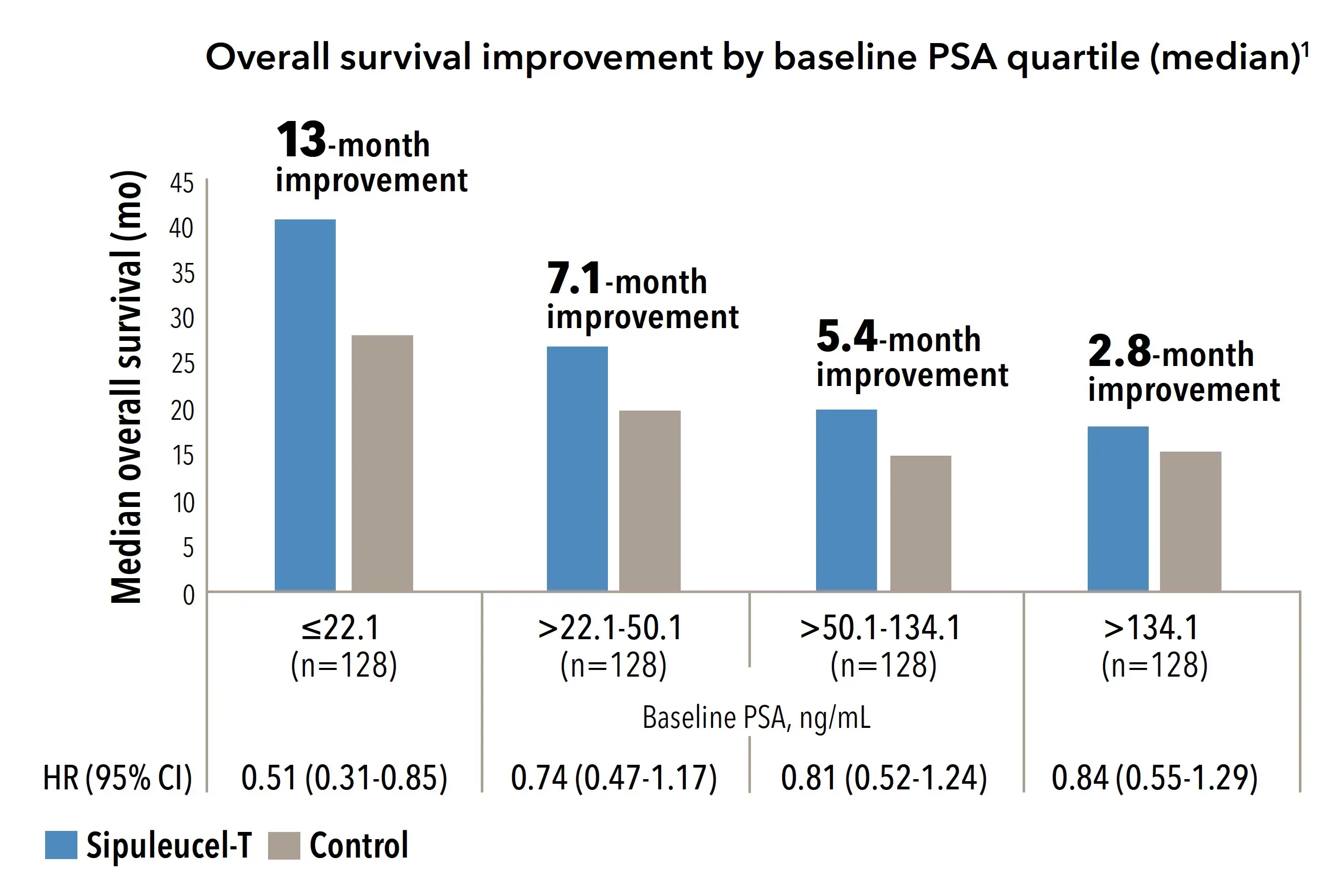
It was this hypothesis that led researchers to reexamine the pivotal IMPACT trial in a post-hoc analysis. A report published in Urology in 2013 examined the association between baseline PSA levels and overall survival benefit (OS). The data from this analysis suggested that men with mCRPC who received sipuleucel-T when their PSA was <22.1 ng/mL, had a median OS improvement of 13 months when compared to untreated men in the same PSA grouping (Figure 1)6.
Figure 1.
Overall Survival Improvement by Baseline PSA Quartile (median)6
This post hoc analysis was not powered for statistical significance, and the population within the subgroups was not randomized. Therefore, the findings are limited by their exploratory nature.
At the time of the IMPACT trial (2003-2007), men with a PSA of <22.1 ng/mL accounted for a mere 25% of the mCRPC population in the study. In those days there was simply no clinical advantage to closely monitoring PSA unless a patient was experiencing cancer-related pain.
In my practice today, PSA values for most patients at the time of mCRPC diagnosis is well below that <22.1 ng/mL level thanks to treatment protocols that require close monitoring of PSA changes, and routine radiographic assessments to ensure we identify the crucial time window when a patient’s cancer metastasizes. When considering immunotherapy for my mCRPC patients, I am thinking about the potential to extend life by more than a year for certain patients, not 4.1 months as originally reported in all cohorts.
There is immense excitement around immunotherapy across various tumor types, however, its use in treating mCRPC remains low, despite a demonstrated survival benefit and generally manageable safety profile. Additionally – and people may not think about this as often as they should – an entire course of PROVENGE therapy is just three infusions in as little as six weeks. We know from earlier data that while immunotherapy takes some time to generate an immune system response, that response can be long lasting. The addition of sipuleucel-T as the foundation of mCRPC treatment provides us with a unique ability to offer therapies with differing mechanisms of action to maximize the available treatment options for our patients. As other specialties wait patiently for immunotherapy to become a clinical reality, urologists have an effective option today, and we should use it.
Michael Fabrizio MD, FACS, is CEO of Urology of Virginia and Professor of Urology at Eastern Virginia Medical School. He specializes in Endourology, including laparoscopic and Robotic Surgery, and advanced prostate cancer therapies such as immunotherapy and oral oncolytics. He is board certified by the American Board of Urology and the National Board of Medical Examiners and is a fellow of the American College of Surgeons. He is a member of the American Urological Association (AUA), Mid-Atlantic Section of the AUA, Endourology Society, and the Society of Urologic Oncology. In addition, he served as the President-elect of the Mid-Atlantic Section of the AUA and is a member of the LUGPA (Large Urology Group Practice Association) board of directors. He has been published extensively in many peer-reviewed outlets and lectures globally.
INDICATION
PROVENGE® (sipuleucel-T) is an autologous cellular immunotherapy indicated for the treatment of asymptomatic or minimally symptomatic metastatic castrate-resistant (hormone-refractory) prostate cancer.
IMPORTANT SAFETY INFORMATION
Acute Infusion Reactions: Acute infusion reactions (reported within 1 day of infusion) may occur and include nausea, vomiting, fatigue, fever, rigor or chills, respiratory events (dyspnea, hypoxia, and bronchospasm), syncope, hypotension, hypertension, and tachycardia.
Thromboembolic Events: Thromboembolic events, including deep venous thrombosis and pulmonary embolism, can occur following infusion of PROVENGE. The clinical significance and causal relationship are uncertain. Most patients had multiple risk factors for these events. PROVENGE should be used with caution in patients with risk factors for thromboembolic events.
Vascular Disorders: Cerebrovascular events (hemorrhagic/ischemic strokes and transient ischemic attacks) and cardiovascular disorders (myocardial infarctions) have been reported following infusion of PROVENGE. The clinical significance and causal relationship are uncertain. Most patients had multiple risk factors for these events.
Handling Precautions: PROVENGE is not tested for transmissible infectious diseases.
Concomitant Chemotherapy or Immunosuppressive Therapy: Chemotherapy or immunosuppressive agents (such as systemic corticosteroids) given concurrently with the leukapheresis procedure or PROVENGE has not been studied. Concurrent use of immune-suppressive agents may alter the efficacy and/or safety of PROVENGE.
Adverse Reactions: The most common adverse reactions reported in clinical trials (≥ 15% of patients receiving PROVENGE) were chills, fatigue, fever, back pain, nausea, joint ache, and headache.
- American Cancer Society. Key Statistics for Prostate Cancer. LINK. Accessed 23July19.
- Dendreon data on file.
- Kantoff PW. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N Engl J Med. Jul2010.
- US FDA. Basis of Approval Letter. LINK. Accessed 23July19.
- Kong HY. Emerging Roles of Human Prostatic Acid Phosphatase. Biomol Ther. Jan2013. LINK. Accessed 23July19.
- Schellhammer PF. Lower baseline prostate-specific antigen is associated with a greater overall survival benefit from sipuleucel-T in the Immunotherapy for Prostate Adenocarcinoma Treatment (IMPACT) trial. Urology. Apr2013.